1. Theory of Chemical Combination
The theory believes that the coupling agent contains a chemical functional group that can interact with the silanol group on the surface of the glass fiber or the molecules on the surface of other inorganic fillers to form a covalent bond; in addition, the coupling agent also contains a different functional group and The polymer molecules are bonded to obtain good interface bonding, and the coupling agent acts as a bridge between the inorganic phase and the organic phase.
The silane coupling agent is taken as an example to illustrate the chemical bond theory. For example, aminopropyltriethoxysilane, when the inorganic filler (such as glass fiber, etc.) is first treated with it, the silane is firstly hydrolyzed into silanol, and then the silanol group undergoes a dehydration reaction with the surface of the inorganic filler to form a chemical bond. The specific process is as follows:
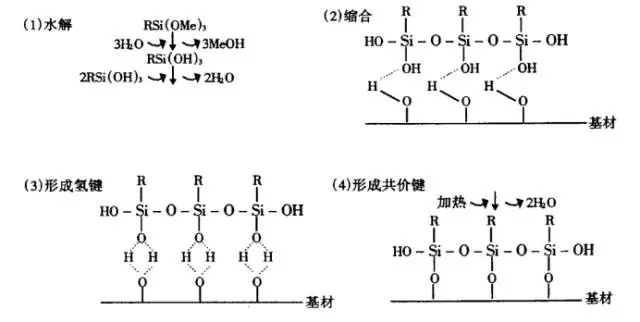
The group in the silane is hydrolyzed-the hydroxyl reacts with the inorganic filler after the hydrolysis-when the inorganic filler treated with the coupling agent is filled to prepare the composite material, the R group in the coupling agent will interact with the organic polymer. Finally, a bridge between inorganic fillers and organics is built.
There are many types of silane coupling agents. The R group in the general formula is different, and the type of polymer that the coupling agent is suitable for is also different. This is because the group R has a selective reaction to the polymer, such as containing vinyl and methyl. Acryloyloxy-based silane coupling agents are particularly effective for unsaturated polyester resins and acrylic resins. The reason is that the unsaturated double bond in the coupling agent and the unsaturated double bond in the resin undergo a chemical reaction under the action of the initiator and accelerator. However, when the coupling agent containing these two groups is used in epoxy resin and phenolic resin, the effect is not obvious, because the double bond in the coupling agent does not participate in the curing reaction of epoxy resin and phenolic resin. However, silane coupling agents with epoxy groups are particularly effective for epoxy resins, and because epoxy groups can react with the hydroxyl groups in unsaturated polyesters, epoxy-containing silanes are also suitable for unsaturated polyesters; Amine-based silane coupling agents are effective for epoxy, phenolic, melamine, polyurethane and other resins. Silane coupling agents containing -SH are widely used in the rubber industry.
2. Wetting effect and surface energy theory
In 1963, when ZISMAN reviewed the known aspects of surface chemistry and surface energy related to adhesion, it was concluded that in the manufacture of composite materials, the good infiltration of the adherend by the liquid resin is of paramount importance. If complete infiltration can be obtained, then the physical adsorption of the resin to the high-energy surface will provide a bonding strength higher than the cohesive strength of the organic resin.
3. Deformable layer theory
In order to alleviate the interfacial stress caused by the difference in thermal shrinkage between the resin and the filler when the composite material is cooled, it is desirable that the resin interface adjacent to the treated inorganic material is a flexible and deformable phase, so that the composite material’s toughness maximum. The surface of the inorganic material treated with the coupling agent may preferentially absorb a certain compounding agent in the resin. The uneven curing of the interphase area may result in a much thicker multi-molecular layer between the polymer and the filler than the coupling agent. Flexible resin layer. This layer is called a deformable layer, which can relax the interface stress and prevent the expansion of interface cracks, thus improving the bonding strength of the interface and improving the mechanical properties of the composite material.
4. Constraint layer theory
In contrast to the deformable layer theory, the constrained layer theory believes that the resin in the inorganic filler area should have a modulus between the inorganic filler and the matrix resin, and the function of the coupling agent is to "tighten the polymer structure". "In the interphase area. From the performance of the reinforced composite material, to obtain the maximum adhesion and hydrolysis resistance, a constraining layer is required at the interface.
As for the titanate coupling agent, its combination with organic polymers in thermoplastic systems and filler-containing thermosetting composites is mainly based on the compatibility and mutual entanglement of long-chain alkyl groups, and forms a co-existence with inorganic fillers. Valence bond. The above hypotheses all reflect the coupling mechanism of the coupling agent from different theoretical sides. In the actual process, it is often the result of the joint action of several mechanisms.
